In this article, I will attempt to answer all these questions and more! So, why is the ozone layer important? The ozone layer protects living beings from harmful ultraviolet radiations that cause severe harmful effects including skin problems, risk of DNA, and other cellular damage. The Ozone (O3) layer is a part of the Stratosphere. It is present in traces in the atmosphere but is good in trapping UV radiations (specifically UVB radiation) and absorbs around 95% of UV-B rays and prevents it from reaching the Earth’s surface. Let’s deep dive into more detail in further subheadings. Stay connected.
How does the Ozone layer protect us? – Benefits of the Ozone layer
The ozone (O3) molecule is made up of three oxygen atoms. It is an allotrope of oxygen. It is different from dioxygen (O2) we need to breathe. It’s a pale blue gas at standard conditions and dark blue liquid at cryogenic temperatures. It is unstable and highly reactive. More powerful oxidant than dioxygen. At concentrations above 100 parts per billion, it’s a potent health hazard and pollutant near the earth’s surface. Ozone near the earth’s surface is known as tropospheric ozone, ground-level ozone, and bad ozone. It is 10% of all the ozone found on the Earth.
It is created by two sources: – • Ozone from the stratosphere gets transferred to the troposphere • Reactions between nitrous oxides and volatile organic compounds (the result of human activities). In the stratosphere, it is known as stratospheric ozone and good ozone. Ozone in the stratosphere is 90% of the planet’s ozone. In the stratosphere, ozone is created by the reaction between UV radiation and dioxygen molecules. O2 + UV = O + O The resultant oxygen atoms are very reactive. Each of them can react with another dioxygen molecule to form an ozone molecule. In this way, the energy of UV radiation is used to break dioxygen molecules and create an ozone layer. O2+ O = O3 The stratospheric good ozone acts as a protective shield for us because it can efficiently absorb harmful UVB radiation resulting in the following benefits: • It protects us from skin diseases • Protect plants from various diseases, helps them indirectly in maintaining their biogeochemical cycles • Maintain population of phytoplankton in the marine ecosystem • Protect many marine animals at their early developmental stages
What Causes Ozone Layer Depletion?
When some chemical compounds generated by industries and human activities reach the stratosphere and get exposed to UV radiation they release chlorine and bromine. These chlorine and bromine start destroying the ozone molecules. It must be noted that the natural process of generation of ozone is far slower than its depletion caused by chemical compounds generated by human activities. One chlorine atom can destroy thousands of ozone molecules. The chemical compounds which cause ozone depletion are called Ozone Depleting Substances (ODS). Check out the detailed chemical structure of chlorine and bromine.
Chlorine Releasing ODS –
• Chlorofluorocarbons (CFCs) • Hydrochlorofluorocarbons (HCFCs) • Carbon Tetrachloride • Methyl Chloroform
Bromine Releasing ODS
• Halons • Methyl bromide
Aerosols released during volcanic eruptions indirectly affect the levels of ozone. These aerosols increase the effectiveness of chlorine in destroying ozone. Some nitrogenous compounds like NO2, N2O, NO are also responsible for ozone layer depletion.
Trends of Ozone Layer Depletion
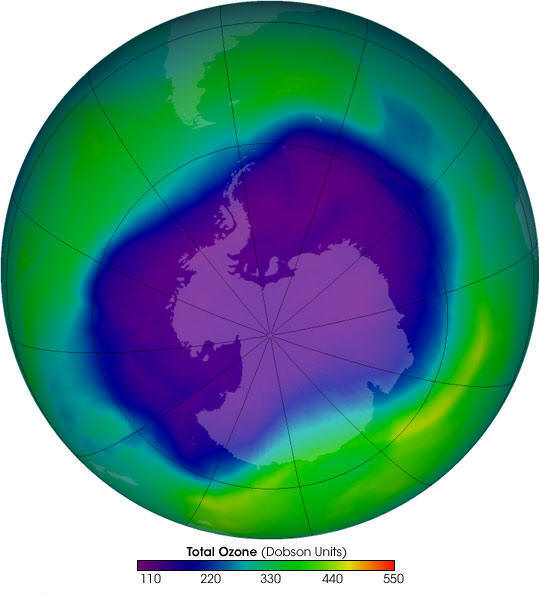
Ozone molecules are constantly created and destroyed, also natural processes like wind and other transport processes keep moving ozone molecules. It causes the concentration of stratospheric ozone to vary naturally throughout the year. However, for several decades human activities have altered the ozone layer. Since the 1970s due to the increased levels of chlorine and bromine in the stratosphere there has been a substantial increase in Ozone depletion, it is more pronounced in polar regions. Dobson Units (DU) is used to measure the concentration of ozone. Dobson unit is the number of molecules required to create a layer of pure ozone that is 0.01 mm thick at 0 degrees Celsius and 1 atmospheric pressure. An ‘Ozone Hole’ is an area with an ozone concentration of around 100 Dobson Units. The South hemisphere concentration of stratospheric ozone from 1979 through to the early 1990s has fallen to the level of 100 DU. For several decades the value has continued to be around 100 DU. But, since 2010, stratospheric ozone concentrations have started to slowly recover because of decisions taken by Montreal Protocol.
What is an Ozone hole and How is an Ozone hole formed?
The ozone hole is the large region of the stratosphere over the Antarctic where the ozone layer is extremely low in amount. It is an annual phenomenon that has started occurring during spring in the Antarctic since 1980. When CFCs from human activities reach the stratosphere, exposure to UV radiation separates the chlorine atom. This chlorine atom reacts with ozone and destroys it while remaining unchanged to cause more damage to the ozone.
The chlorine released from CFCs later becomes part of two chemical compounds – hydrochloric acid and chlorine nitrate. During dark winters of the antarctic chemical reactions taking place in the stratosphere releases chlorine (Cl2) from the two compounds. In October when sunlight return to the south pole, UV radiation breaks releases the chlorine atoms, which again destroys thousands of ozone molecules.
What are UVA and UVB radiations? Which one is more harmful?
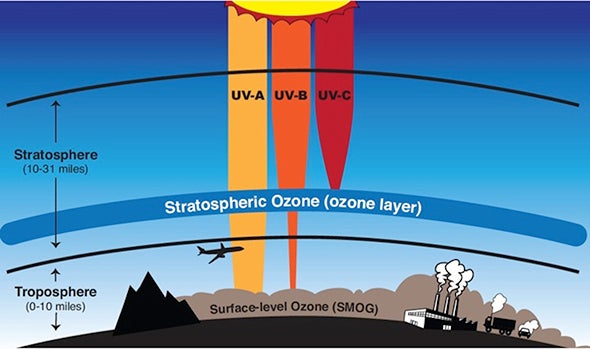
Ultraviolet or UV radiations are electromagnetic waves of wavelength shorter than visible light therefore invisible to our eyes. Prolonged unprotected exposure to UV can produce genetic defects in skin cells. They can also damage our eyes. Two types of UV radiations from the sun reaches the earth surface- • UVA which has a longer wavelength and lower energy (~95% of the Sun UV). • UVB which has a shorter wavelength and higher energy (~5% of the Sun UV). UVA can go deeper into the skin. UVB affects the top layer of skin and can cause skin burn. It must be noted that long-duration exposure to any kind of UV is equally harmful to us.
UVB has other harmful effects – • It affects many physiological and developmental processes of plants. • It affects the population of phytoplankton. • It damages the early developmental stage of many marine animals. • It can break down many synthetic and naturally occurring polymers of commercial interest.
How to prevent Ozone depletion?
The international community become concerned to protect the ozone layer from ODS during the 1970s and 1980s. The Vienna Convention for the Protection of the Ozone Layer formalized international cooperation on protecting stratospheric ozone. This leads to the signing of the Montreal Protocol in 1987 by 149 countries. The first data after the Montreal Protocol was signed, showed greater damage to the ozone layer. Therefore in 1992, as a countermeasure changes were made in the terms of the original 1987 agreement to end production of halons by 1994 and CFCs by 1996 in developed countries.
The decisions made by Montreal Protocol have resulted in a decrease in the emission of ODS. It is expected that near the middle of the 21st century and the ozone layer will be fully healed. Ozone depletion can be prevented by the following steps- • Reduce the use of ODS for example CFCs in refrigerators and air conditioner coolants. • Minimal use of vehicles. • Find a substitute for cleaning products because most of them contain chlorine and bromine. • There should be a prohibition against the use of nitrous oxides.
Conclusion
Based on the above discussion it can be concluded that the ozone layer is the protective shield required for the survival of living organisms on earth. It absorbs ~95% of the Sun’s harmful UV radiation. Therefore, in the absence of the ozone layer, we would be more susceptible to many health hazards. Every year 16th of September is celebrated as World Ozone Day. This day is used to spread awareness among the people regarding the depletion of the ozone layer and its harmful effects. Happy learning!!