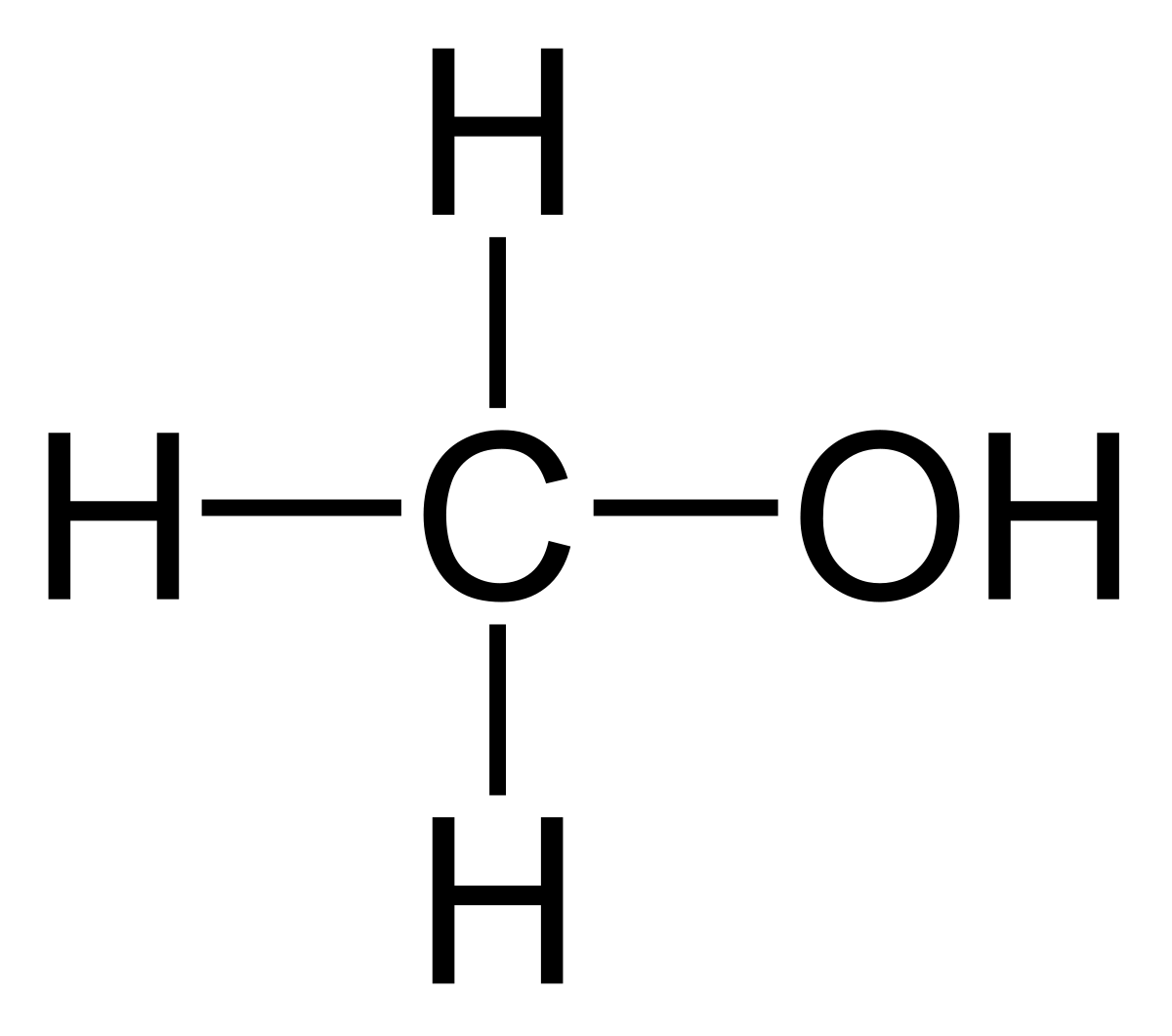
The term alcohol was originally used for ethanol (CH3CH2OH). The general formula for alcohol is CnH2n+1OH. Alcohols are used for a number of purposes like methanol is used for the preparation of formaldehyde, ethanol is widely used as a beverage and solvent, many fatty alcohols are also used in the preparation of detergents. The alcohols have a higher boiling point due to the formation of intermolecular hydrogen bonding owing to the presence of the hydroxyl group. If more than one hydroxyl groups are present the alcohols are termed as diols, triols, etc. So, is alcohol soluble in water? Yes, Alcohols are soluble in water owing to the presence of the hydroxyl (-OH) group, which is hydrophilic (water-loving). The alcohol molecules form hydrogen bonds with water molecules enhancing the solubility of alcohol in water. The solubility of alcohol in water decreases as the number of carbon atoms increases. Also, the solubility of alcohol in water increases as the number of hydroxyl groups increases.
Why are Alcohols Soluble in Water?
Water molecules are polar and form intermolecular hydrogen bonds amongst them. They are also capable of forming hydrogen bonding with other similar molecules. In the case of alcohol, the slightly negative oxygen atom of the hydroxyl functional group (-OH) is attracted to the slightly positive hydrogen atom of the water molecule and immediately forms a hydrogen bond. Also, both alcohol and water are polar liquids due to which they tend to blend easily. In order of polarity, alcohols are ranked third after amides and acids due to their capacity of formation of hydrogen bonds and the presence of an oxygen atom in alcohol molecules. However, the solubility of alcohol in water decreases as the length of the carbon chain increases. The reason for this is the presence of intermolecular hydrogen bonding between alcohol molecules due to which the alcohol molecules remain closely packed with each other as the size and mass increase. A large amount of energy is required to break these hydrogen bonds. In the case of small chain alcohol (up to 4 molecules) the energy required to break the hydrogen bond between alcohol molecules is compensated with the energy released on the formation of new hydrogen bonds between alcohol and water molecules. However, in the case of long-chain alcohol molecules, a number of hydrogen bonds are broken to accommodate the bulky alkyl group which cannot be compensated as the alkyl groups do not form hydrogen bonds. Another reason for decreasing solubility with increasing mass is that the alkyl group of alcohol is non-polar and hydrophobic (water-hating) and pushes the water molecules away from the alcohol molecules. With the increase in length of the carbon chain, the proportion of the alkyl group increases in alcohol molecules due to which the solubility decreases, and post four-carbon chain the alcohol and water solution can be seen as two immiscible liquids forming two different phases.
Which Alcohol is most Soluble in Water?
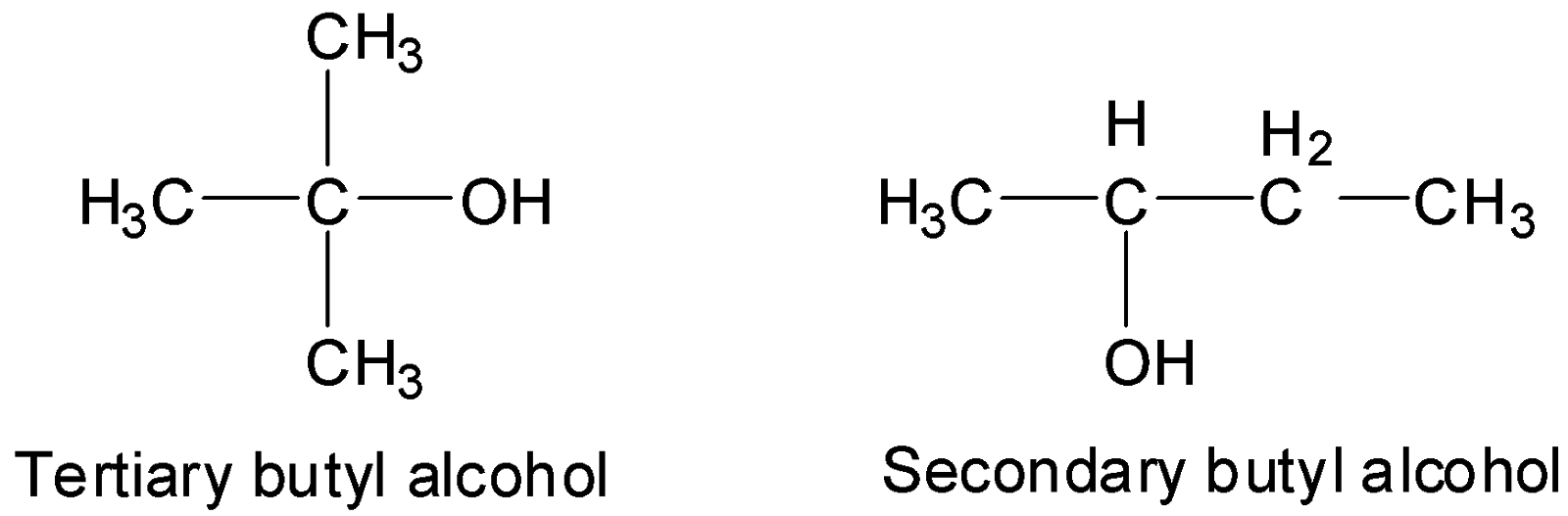
As discussed in the earlier section the solubility of alcohols in water decreases as the number of carbon atoms increases. Methanol has the smallest alkyl group out of all the alcohols due to which it is most soluble in water. However, this is to be noted that both ethanol and methanol are completely soluble in water at room temperature. So, how do we decide that which one is more soluble? The answer is by the ease of separation of the mixture. Once the alcohol-water mixture is formed salt is added to separate the two liquids (a process called salting). The ethanol molecules separate out easily in comparison to methanol molecules, indicating that the methanol molecules are more closely bound with water molecules and thus, are more soluble in water. Amongst primary alcohols, the first three i.e. methanol, ethanol, and propanol are miscible in water at room temperature. The solubility limits with the decrease in temperature. The trend that solubility of alcohol decreases with an increase in the length of the carbon chain is applicable for primary alcohols. On the other hand in the case of isomeric alcohols, the solubility is affected by the branching of the alkyl group in the alcohol molecule. Usually, solubility increases with an increase in branching. This is because the surface area of the non-polar alkyl group is lesser exposed to water molecules resulting in ease of formation of hydrogen bonds. Therefore, the solubility order for isomeric alcohols is: Tertiary > Secondary > Primary Hence, it can be deduced that when it comes to bulky groups the branched-chain alcohols are more soluble than the linear chain alcohols.
Solubility of Primary, Secondary, and Tertiary Alcohols in water
Amid primary alcohols the order of solubility is Methanol > Ethanol > Propanol However, from butanol onwards, the solubility of alcohol in water is greatly affected and the bulky linear chain alcohol molecules tend to form separate layers from water when the two are dissolved together. On the other hand in the case of branched-chain alcohols, the bulky groups are still soluble and the order of solubility for isomeric alcohols is: Tertiary > Secondary > Primary The reason for this is that in the case of a primary alcohol, owing to the presence of a bulky alkyl group (hydrophobic), most water molecules are far away from the hydroxyl group and are not available for hydrogen bonding. However, the water molecules have a chance to remain relatively closer in the case of secondary alcohols where the alkyl group is branched and affects lesser water molecules, comparatively. The probability of water molecules getting closer to the hydroxyl group further increases in the case of tertiary alcohols as the branching increases.
Solubility Chart for Alcohols
The alcohols consist of a polar group viz. hydroxyl functional group (-OH group) which is hydrophilic and a non-polar group viz. alkyl group which is hydrophobic. Therefore, the solubility of alcohol in water is determined by the strength of these two forces acting against each other. The solubility is measured in mol/100g of H2O at 1atm and 25 degrees Celcius.
Factors Affecting the Solubility of Alcohols
The solubility is the property of a substance to dissolve in another substance. The substance here can be solid, liquid, or gas. It depends upon the physical and chemical properties of the substance. The substances with similar kinds of intermolecular forces of attraction tend to be better soluble with each other. In the case of alcohols the solubility is dependent on a number of factors, which are:
• The extent of hydrogen bonding between alcohol and water molecules
As the alcohol molecules dissolve in water due to the formation of hydrogen bonding, the extent of hydrogen bond plays a major role.
• The strength of hydrophobic and hydrophilic forces
The hydrophobic part (alkyl group) of alcohol pushes away the water molecules while the hydrophilic part (hydroxyl group) bonds with the water molecule. The solubility depends upon which of these forces are stronger.
• The length of the carbon chain
The alcohol molecule with a small number of carbon atoms dissolves better in water in comparison to bulky or long-chain alkyl groups. Due to this, the methanol molecules are most soluble in water while butanol and other alcohols with long carbon chains tend to form separate layers when dissolved in water.
• Branching of the alkyl group
The branched-chain alkyl groups offer a lesser surface area exposed to water molecules in comparison to linear chain alkyl groups. As the alkyl groups are hydrophobic, it means that the extent of hydrophobic forces diminishes with the branching of the alkyl group in the alcohol molecule owing to which the solubility of alcohol in water increases. Below are the images of branched alcohols.
• The number of the hydroxyl groups present
The hydroxyl group (-OH) present in the alcohol molecules is responsible for the formation of hydrogen bonding between alcohol and water molecules. Therefore, the solubility of alcohol in water increases with the number of hydroxyl groups. This is the reason that diols are more soluble in water in comparison to similar alcohols.
Conclusion
• Alcohols are soluble in water due to the formation of hydrogen bonding between alcohol and water molecules and the polar nature of both liquids. • The hydroxyl group, owing to its hydrophilic nature and capacity of hydrogen bond formation, is responsible for the solubility of alcohols in water. • Amongst primary alcohols, the order of solubility is Methanol > Ethanol > Propanol. • In the case of isomeric alcohol the order of solubility is Tertiary > Secondary > Primary • The extent of hydrogen bonding, the strength of hydrophobic and hydrophilic forces, length of the carbon chain, branching of the alkyl group, and the number of hydroxyl groups present in the alcohol molecule determine its solubility in water. So, this was all about the solubility of alcohols in water. I hope you guys found the article informative. Please share it in your circle and let me know any suggestions by comments. Thanks!!